General Research Concept
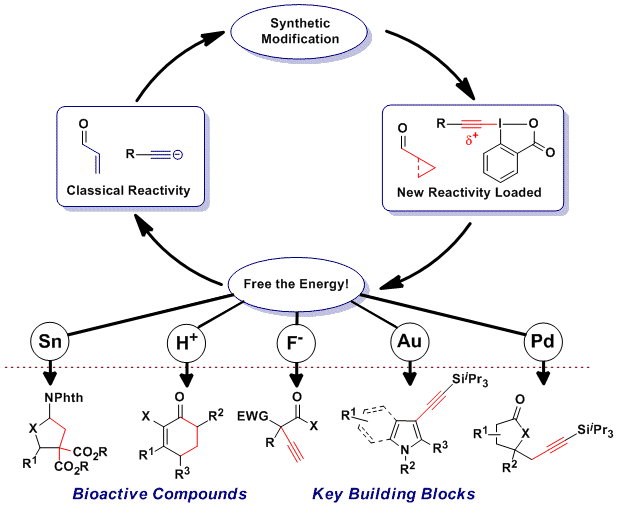
The development of new non-conventional organic synthons stands at the center of our research. We harness the power of modern catalysis to promote C-C or C-X bond formation, allowing for a rapid and efficient enhancement in molecular complexity. In order to access unprecedented reactivity, we use a two steps approach:
1) Synthetic Modification: Classical functional groups are modified to create high energy entities, such as strained rings and hypervalent iodine compounds with a new reactivity profile.
2) Catalytic Activation: The potential reactivity of the intermediates is freed under the control of a catalyst to generate new bonds under mild conditions.
In case the substrate reactivity is too low, we recently developed new in situ tethering approaches exploiting existing functional groups.
The developed methods give access to important natural and synthetic bioactive compounds, and set the stage for new applications in chemical biology and material sciences
Current Research Area in the Group:
Project 1: Electrophilic alkynylation with and without hypervalent iodine reagents
Project 2: Transformations beyond alkynylation using hypervalent iodine reagents
Project 3: Modification of peptides and proteins
Project 4: Cyclization and annulation reactions initiated by the opening of small rings
Project 5: In situ tethering strategies for the functionalization of olefins and alkynes
Stability Data of Benziodoxole Reagents
For a recent personal account about the background behind the discovery of benziodoxole reagents in our laboratory, see: Acc. Chem. Res. 2018, 51, 3212-3225. (DOI: 10.1021/acs.accounts.8b00468).